Chemical Education
Ready to bring the Organic Lab into the 21st Century?
In order to maintain a competitive edge in research and technology it is imperative that undergraduates are taught state of the art chemistry laboratory techniques. Owing to recent developments in organic chemistry, including Solid Phase Organic Chemistry (SPOC), the traditional Organic Chemistry Laboratory class is lacking in content to adequately prepare chemistry graduates for direct entry into the workforce.
Due in large part to the development and use of SPOC, combinatorial chemistry has become one of the major tools in the search for lead compounds in drug discovery.[i] In SPOC, a reagent is “immobilized” on a solid support, such as polymeric beads made of polystyrene or silica gel. Two general protocols have been used in SPOC:
1. The use of polymer scaffolds for construction of target compounds in a multi-step sequence.
2. The use of polymer-supported reagents to mediate a single synthetic step.
The use of solid supports for building complex molecules has become routine in well-known applications including peptide[ii] and oligonucleotide synthesis[iii]. More recently, the ability to efficiently prepare amides from carboxylic acids via commercially available activating agents immobilized on polystyrene and silica gel supports (e.g. 1-hydroxybenzotriazole (HOBt), dichlorotriazine (DCT), carbodiimide) expands the synthetic chemist’s SPOC toolbox.[iv]
Amides have applications in biochemistry[v], medicinal chemistry[vi], and other fields such as agrochemistry[vii]. Unfortunately, amide synthesis is an area of organic chemistry that is not well represented in modern undergraduate laboratory manuals. Few examples are available, all of which employ solution phase methodology.[viii],[ix] Typically, the reaction between an acid chloride and a primary amine is performed in solution, followed by isolation and purification via acid-base extraction. Published undergraduate laboratory experiments follow this general protocol.
The availability of easily accessible SPOC protocols and reagents for amide synthesis provides a unique opportunity to not only bring SPOC into the Organic Laboratory curriculum but also re-introduce the preparation of amides to the undergraduate laboratory experience.
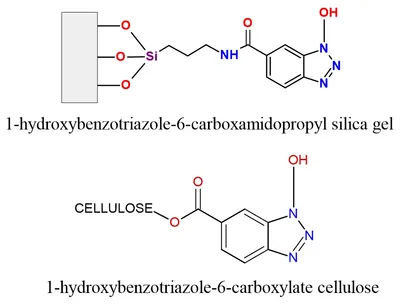
Aurora has developed novel functionalized solid phase reagents that not only jump start this process but also bring innovation to the undergraduate laboratory. Si-HOBt (Catalog # H 1002, 1-hydroxybenzotriazole-6-carboxamidopropyl silica gel) and Cellulose-HOBt (Catalog # H 1002-DSK-05, 1-hydroxybenzotriazole-6-carboxamidopropyl cellulose) are utilized for the formation of immobilized activated ester and sulfonate ester reagents for the facile formation of amides and sulfonamides, respectively. The immobilized HOBt is activated utilizing standard coupling chemistry with carboxylic acids (or sulfonyl chlorides) followed by reaction with amines to generate high yields of pure amide and sulfonamide products after filtration. Immobilized HOBt is quite suitable for combinatorial synthesis applications. The used Si-HOBt is readily regenerated and can be recycled repeatedly for further use.[x]
The use of immobilized HOBt in the Organic Laboratory class is the ideal way of introducing SPOC to future organic chemists. Due to its ease of use and ability to be recycled, Si-HOBt updates the Organic Chemistry Lab class in an effective and economical way. Straightforward experiments designed around amide and sulfonamide formation can be readily incorporated into the curriculum.
[i] Houghten, R. A.; Yongping, Y. J. Am. Chem. Soc. 2005, 127, 8582.
[ii] Merrifield, R. J. Am. Chem. Soc. 1963, 85, 2149.
[iii] Brown, T; Brown, D.J.S. In Oligonucleotides and Analogues, A Practical Approach; Eckstein, F., ed.; IRL Press: Oxford, 1991, p. 1-24.
[iv] Vokkaliga, S.; Jeong, J.; LaCourse, W. R.; Kalivretenos, A. Tetrahedron Letters. 2011, 52, 2722 – 2724.
[v] Nelson, D. L. and Cox, M. M. Lehninger Principles of Biochemistry, 5th ed.; Freeman: New York, 2008.
[vi] Kimachi, T. and Takemoto, Y. J. Org. Chem. 2001, 66, 2700 – 2704.
[vii] Swithenbank, C.; McNulty, P. J.; Viste, K. L. J. Agr. Food Chem. 1971, 19, 417 – 421.
[viii] Wilcox, Jr. C. F. and Wilcox, M. F. Experimental Organic Chemistry: A Small Scale Approach, 2nd Ed.; Prentice-Hall: Englewood Cliffs, NJ, 1995.
[ix] Landgrebe, J. A. Theory and Practice in the Organic Laboratory, 5th ed.; Brooks/Cole: Belmont, CA, 2005.
[x] a) Dendrinos, K. et al (1998) Chem Comm, 499.b) Dendrinos, K., Kalivretenos, A.G. (1998) J. Chem Soc Perkin Trans I, 1463. c) Dendrinos, K., Kalivretenos, A.G. (1998) Tetrahedron Lett, 39, 1321. d) Kalivretenos, A.G. US Patent # 7,229,835. e) Vokkaliga, S. et al (2011) Tetrahedron Lett., 52, 2722.